The idea of a physical quantity is complete only when it is measured. The need to measure PV arose at an early stage of knowledge of nature and increased with the development and complexity of human production and scientific activities. Requirements for the accuracy of EF measurements are constantly increasing.
Measure a physical quantity- means comparing it with a homogeneous quantity, conventionally accepted as a unit of measurement.
There are two ways to measure an unknown physical quantity:
A) Direct measurement called a measurement in which the value of PV is determined directly from experience. Direct measurements include, for example, measuring mass with a scale, temperature with a thermometer, and length with a scale ruler.
b) Indirect measurement is a measurement in which the desired PV value is found by direct measurement of other PVs based on a known relationship between them. An indirect measurement is, for example, determining the density ρ substances by direct volume measurements V and masses m bodies.
Specific implementations of the same PV are called homogeneous quantities. For example, the distance between the pupils of your eyes and the height of the Ostankino tower are specific realizations of the same PV - length and therefore they are homogeneous quantities. The mass of a cell phone and the mass of a nuclear icebreaker are also homogeneous physical quantities.
Homogeneous PVs differ from each other in size. The size of the PV is the quantitative content in a given object of a property corresponding to the concept of “physical quantity”. The sizes of homogeneous physical quantities of different objects can be compared with each other.
Let us emphasize the significant difference between physical quantities and units of their measurement. If the measured PV value answers the question “how much?”, then the unit of measurement answers the question “what?” Some units of measurement can be reproduced in the form of some kind of bodies or samples (weights, rulers, etc.). Such samples are called measures. Measurements carried out with the highest accuracy currently achievable are called standards.
The value of a physical quantity is an assessment of a physical quantity in the form of a certain number of units accepted for it. Basic units of measurement are arbitrary units of measurement for a few quantities (independent of each other), with which all others are in a certain connection. It is necessary to distinguish true And real values of a physical quantity.
True meaning EF is the ideal value of EF, existing objectively regardless of the person and the methods of its measurement. However, the true meaning of PV is, as a rule, unknown to us. And it can only be known approximately with a certain accuracy by measurement.
Real value PV is a value found experimentally – by measurement. The degree of approximation of the actual value of the PV to the true one depends on the perfection of the technical measuring instruments used.
EF measurements are based on various physical phenomena. For example, the thermal expansion of bodies is used to measure temperature, the phenomenon of gravity is used to measure the mass of bodies by weighing, etc. The set of physical phenomena on which measurements are based is called measuring principle .
Measuring instruments include measures, measuring instruments, etc.
Measuring device is a measuring instrument designed to generate a signal of measuring information in a form accessible to direct perception by a person. Measuring instruments include ammeter, dynamometer, ruler, scales, pressure gauge, etc.
In addition to the basic physical quantities in physics, there are derived physical quantities that can be expressed through the basic ones. To do this, it is necessary to introduce two concepts: the dimension of the derivative quantity and the defining equation. Derived units are obtained from the basic ones using equations of connection between the corresponding quantities.
Sensitivity of measuring instruments – Measuring instruments are characterized by sensitivity. The sensitivity of the measuring device is equal to the ratio of the linear (Dl) or angular (Da) movement of the signal pointer on the scale of the device to the change DX of the measured value X that caused it. Sensitivity determines the minimum measured PV value using this device.
Classification of measuring instruments can be carried out according to the following criteria.
1. Accuracy characteristics measurements are divided into equal and unequal.
Equal-precision measurements a physical quantity is a series of measurements of a certain quantity made using measuring instruments (MI) with the same accuracy under identical initial conditions.
Unequally accurate measurements a physical quantity is a series of measurements of a certain quantity made using measuring instruments with different accuracy and (or) under different initial conditions.
2. By number of measurements measurements are divided into single and multiple.
Single measurement is a measurement of one quantity made once. In practice, single measurements have a large error; therefore, to reduce the error, it is recommended to perform measurements of this type at least three times, and take their arithmetic average as the result.
Multiple measurements is a measurement of one or more quantities performed four or more times. A multiple measurement is a series of single measurements. The minimum number of measurements at which a measurement can be considered multiple is four. The result of multiple measurements is the arithmetic average of the results of all measurements taken. With repeated measurements, the error is reduced.
3. By type of change in value measurements are divided into static and dynamic.
Static measurements- These are measurements of a constant, unchanging physical quantity. An example of such a time-constant physical quantity is the length of a land plot.
Dynamic measurements– these are measurements of a changing, non-constant physical quantity.
4. By purpose measurements are divided into technical and metrological.
Technical measurements – These are measurements performed by technical measuring instruments.
Metrological measurements are measurements made using standards.
5. By way of presenting the result measurements are divided into absolute and relative.
Absolute measurements– these are measurements that are performed through direct, direct measurement of a fundamental quantity and (or) the application of a physical constant.
Relative measurements- these are measurements in which the ratio of homogeneous quantities is calculated, with the numerator being the quantity being compared, and the denominator being the basis of comparison (unit). The measurement result will depend on what value is taken as the basis of comparison.
6. By methods of obtaining results measurements are divided into direct, indirect, cumulative and joint.
Direct measurements– these are measurements performed using measures, i.e. the measured quantity is compared directly with its measure. An example of direct measurements is the measurement of an angle (measure - protractor).
Indirect measurements are measurements in which the value of the measurand is calculated using values obtained through direct measurements and some known relationship between these values and the measurand.
Aggregate Measurements– these are measurements, the result of which is the solution of a certain system of equations, which is composed of equations obtained as a result of measuring possible combinations of measured quantities.
Joint measurements – These are measurements during which at least two inhomogeneous physical quantities are measured in order to establish the relationship that exists between them.
4. Units of measurement
In 1960, at the XI General Conference on Weights and Measures, the International System of Units (SI) was approved.
The International System of Units is based on seven units covering the following fields of science: mechanics, electricity, heat, optics, molecular physics, thermodynamics and chemistry:
1) unit of length (mechanics) – meter;
2) unit of mass (mechanics) – kilogram;
3) unit of time (mechanics) – second;
4) unit of electric current (electricity) – ampere;
5) unit of thermodynamic temperature (heat) – kelvin;
6) unit of luminous intensity (optics) – candela;
7) unit of quantity of a substance (molecular physics, thermodynamics and chemistry) – mole.
There are additional units in the International System of Units:
1) unit of measurement of a plane angle – radian;
2) unit of measurement of solid angle – steradian Thus, through the adoption of the International System of Units, units of measurement of physical quantities in all fields of science and technology were streamlined and brought to one type, since all other units are expressed through seven basic and two additional SI units. For example, the amount of electricity is expressed in terms of seconds and amperes.
Main measurement characteristics
The following main measurement characteristics are distinguished:
1) the method by which measurements are taken;
2) measurement principle;
3) measurement error;
4) measurement accuracy;
5) correctness of measurements;
6) reliability of measurements.
Measurement method- this is a method or a set of methods by which a given quantity is measured, i.e., a comparison of the measured quantity with its measure according to the accepted principle of measurement.
There are several criteria for classifying measurement methods.
1. According to the methods of obtaining the desired value of the measured quantity, the following are distinguished:
1) direct method (carried out using direct, direct measurements);
2) indirect method.
2. According to measurement techniques, there are:
1) contact measurement method;
2) non-contact measurement method. Contact measurement method based on direct contact of any part of the measuring device with the measured object.
At non-contact measurement method the measuring device does not come into direct contact with the object being measured.
3. According to the methods of comparing a quantity with its measure, the following are distinguished:
1) direct assessment method;
2) method of comparison with its unit.
Direct assessment method is based on the use of a measuring device that shows the value of the measured quantity.
Comparison method with measure based on comparing the object of measurement with its measure.
Measuring principle– this is a certain physical phenomenon or their complex on which the measurement is based. For example, temperature measurement is based on the phenomenon of expansion of a liquid when it is heated (mercury in a thermometer).
Measurement error is the difference between the result of measuring a quantity and the real (actual) value of this quantity. Error, as a rule, arises due to insufficient accuracy of measurement tools and methods or due to the inability to provide identical conditions for repeated observations.
Accuracy of measurements– this is a characteristic that expresses the degree of correspondence of the measurement results to the real value of the measured quantity.
Quantitatively, the measurement accuracy is equal to the relative error minus the first power, taken modulo.
Correct measurement– this is a qualitative characteristic of a measurement, which is determined by how close to zero the value of a constant or fixed error that changes during repeated measurements (systematic error). This characteristic usually depends on the accuracy of the measuring instruments.
The main characteristic of measurements is the reliability of the measurements.
Reliability of measurements is a characteristic that determines the degree of confidence in the obtained measurement results. According to this characteristic, measurements are divided into reliable and unreliable. The reliability of measurements depends on whether the probability of deviation of the measurement results from the real value of the measured value is known. If the reliability of the measurements is not determined, then the results of such measurements, as a rule, are not used. The reliability of measurements is limited above by the error
Measurement- finding the value of a physical quantity experimentally using special technical means.
From the term “Measurement” comes the term “to measure”. Other terms should not be used - “measure”, “measure”, “measure”, “measure”. They do not fit into the system of metrological terms.
To carry out a measurement, it is necessary to have: a physical quantity; measurement method; measuring instruments; operator; conditions required for measurement.
The purpose of measurement is to obtain the value of a physical quantity in a form that is most convenient for use.
What is meant by a physical quantity whose value is found experimentally?
Physical quantity, as noted above, this is a characteristic of a physical object (physical system, phenomenon or process), common in qualitative terms for many physical objects, but quantitatively individual for each of them.
Individuality is understood in the sense that a property can be a certain number of times greater or less for one object than for another object. Examples of physical quantities include density, melting point, refractive index of light, and many others.
A physical quantity is characterized by size, value, numerical value, true and real values.
Size of physical quantity - quantitative determination of a physical quantity inherent in a specific material object, system, phenomenon or process.
The value of a physical quantity is expression of the size of a physical quantity in the form of a certain number of units accepted for it.
Numerical value of a physical quantity- an abstract number included in the value of a quantity.
“Size” is a multi-species concept. But the term “quantity” often expresses the size of a specific physical quantity. It is incorrect to say “magnitude of speed”, “magnitude of voltage”, since both speed and voltage are quantities.
There is a difference between size and magnitude. The size of the quantity really exists. You can express the size of a quantity using any of the units of a given quantity using a numerical value. The numerical value changes depending on the units chosen, while the physical size of the quantity remains the same.
Unit physical quantity- a physical quantity of a fixed size, which is conditionally assigned a numerical value equal to 1.
A physical quantity is characterized by its true meaning which ideally reflects the corresponding property of the object in qualitative and quantitative terms.
Valid called meaning physical quantity, found experimentally and so close to the true value that for this purpose it can be used instead.
Kinds measurements. By method of obtaining Based on the numerical value of the measured quantity, all measurements are divided into four main types: direct, indirect, cumulative and joint.
Direct are measurements in which the desired value of a physical quantity is obtained directly from experimental data (for example, measuring mass on a scale, the length of a part with a micrometer).
Strictly speaking, measurement is always direct and is considered as a comparison of a quantity with its unit. In this case, it is better to use the term “direct measurement method”.
Indirect measurements - determination of the desired value of a physical quantity based on the results of direct measurements of other physical quantities that are functionally related to the desired quantity.
Indirect measurements are carried out in cases where:
* the value of the measured quantity is easier to find by indirect measurements than by direct measurements;
* there are no direct measurements of this or that value;
* indirect measurements give less error than direct measurements.
Equation of indirect measurements: y = f (x (, x 2,... x n), where y is the desired value, which is a function of the arguments x, x 2,..., x n, obtained by direct measurements.
An example of indirect measurements is the determination of hardness (HB) of metals by pressing a steel ball of a certain diameter (D) with a certain load (P) and obtaining a certain indentation depth (h): HB = P/(tcD h).
Cumulative are called simultaneous measurements of several quantities of the same name, in which the values of the desired quantities are found by solving a system of equations obtained from direct measurements.
For example, measurements in which the masses of individual weights in a set are found from the known mass of one of them and from the results of direct comparisons of the masses of various combinations of weights.
Joint measurements - These are measurements made simultaneously of two or several quantities of different names to find the functional relationship between them. For example, determining the dependence of body length on temperature, boiling and melting points on pressure, etc.
Measurements can be classified:
a) according to the accuracy characteristic - equally accurate(a series of measurements of any quantity performed by measuring instruments of equal accuracy and under the same conditions) and unequal(a series of measurements of any quantity made by several
measuring instruments with different accuracy and (or) in several different conditions);
b) by the number of measurements in a series of measurements - one-time And many multiples;
c) in relation to the change in the measured value - static(measurement of a physical quantity that does not change over time, for example, measuring the length of a part at normal temperature or measuring the size of a plot of land) and dynamic(measurement of a physical quantity that varies in size, for example,
measurement of alternating voltage electric current, measurement
distance to ground level from a descending aircraft);
d) by expressing the measurement result - absolute(a measurement based on direct measurements of quantities and (or) the use of values of physical constants, for example, the measurement of force F is based on the measurement of the basic quantity of mass m and the use of a physical constant - the acceleration of gravity g) and relative(measurement of the ratio of a quantity to a quantity of the same name, which acts as a unit).
You can measure the composition or property of substances or measure a physical quantity using one or another measurement method.
Method of measurement- this is a technique or a set of techniques for comparing the measured composition or property of a substance or a measured physical quantity with a known composition or property of a substance or with a unit of physical quantity in accordance with the implemented measurement principle.
Measuring principle- this is the phenomenon or effect underlying the measurements.
Let's look at some of the principles that underlie measurements.
If you heat the junctions of two electrodes made of various materials, an emf occurs. This phenomenon is the basis for high-precision temperature measurement (thermocouples).
When electrical conductors and semiconductors are heated, their resistance changes. This phenomenon makes it possible to obtain highly accurate temperature measurements, especially when using platinum. The use of semiconductors makes it possible to measure small temperature ranges and the temperature of bodies with very small volumes.
When some materials are stretched or compressed, their electrical resistance changes, which is the basis for measuring small deformations of bodies, as well as high and ultra-high pressures. At the interface of a metal and a semiconductor, when illuminated, an emf occurs, the so-called photoelectric effect. Photocells, which are used in many measuring instruments, are based on the use of the photoelectric effect.
The brightness of the glow of the body depends on the temperature, which, in turn, depends on the strength of the current heating the body. A non-contact temperature measurement method (optical pyrometer) is based on this phenomenon.
Lecture 3. MEASUREMENTS OF PHYSICAL QUANTITIES
3.1 Measurements of physical quantities and their classification
3.2 Principles, measurement methods
3.3. Measurement procedure
Measurements of physical quantities and their classification
The reliability of measurement information is the basis for analysis, forecasting, planning and production management in general, helps to increase the efficiency of accounting for raw materials, finished products and energy costs, as well as improve the quality of finished products.
Measurement- a set of operations performed to determine the quantitative value of a quantity;
Measurement of a physical quantity – a set of operations for the use of a technical device that stores a unit of physical quantity, ensuring that the relationship of the measured quantity with its unit is found and the value of this quantity is obtained.
Object of measurement– a real physical object, the properties of which are characterized by one or more measured PVs.
measuring technology– a set of technical means used to perform measurements.
The main consumer of measuring equipment is industry. here, measuring technology is an integral part of the technological process, as it is used to obtain information about technological regimes that determine the course of processes.
technological measurements– a set of measuring devices and measurement methods used in technological processes.
Object of measurement– a body (physical system, process, phenomenon, etc.), which is characterized by one or more measurable or measurable physical quantities.
Measurement quality is a set of properties that determine the compliance of means, method, methodology, measurement conditions and the state of measurement unity with the requirements of the measurement task.
Measurements are classified according to the following criteria:
3.1.1 According to the dependence of the measured value on time into static and dynamic ;
Static measurements– measurement of a physical quantity that, in accordance with the measurement task, is accepted as constant throughout the measurement time (for example, measuring the size of a part at normal temperature).
Dynamic measurements– measurements of a physical quantity whose size changes over time (for example, measurement of the mass fraction of water in a product during the drying process).
3.1.2 By method of obtaining results into direct, indirect, cumulative, joint;
Direct measurement– a measurement in which the desired value of a physical quantity is found directly from experimental data. In the process of direct measurement, the measurement object is brought into interaction with the measuring instrument and, according to the readings of the latter, the value of the measured quantity is measured. Examples of direct measurements include measuring length with a ruler, mass with a scale, temperature with a glass thermometer and active acidity with a pH meter, etc.
Direct measurements include measurements of the vast majority of parameters of a chemical technological process.
Indirect measurement– a measurement in which the desired value of a quantity is found on the basis of a known relationship between this quantity and quantities obtained by direct measurement.
Indirect measurements are used in two cases:
· there is no measuring instrument for direct measurements;
· Direct measurements are not accurate enough.
When conducting chemical analyzes of the composition and properties of food substances, indirect measurements are widely used. An example of indirect measurements can be measurements of the density of a homogeneous body by its mass and volume; determination of the mass fraction of water in fish products by drying at a temperature of 105 O C, the essence of which is to dry the product to a constant mass and determine the mass fraction of water according to the formula:
where M 1 – weight of the weighing bottle with a sample before drying, g; M 2 – weight of the weighing bottle with a sample after drying, g; M is the mass of the sample.
Cumulative measurements – measurements of several homogeneous quantities, in which the required values of the quantities are found by solving a system of equations obtained by direct measurements of various combinations of these quantities (measurements in which the mass of individual weights of a set is found from the known mass of one of them and from the results of direct comparisons of the masses of various combinations of weights).
Joint measurements – simultaneous measurements of two or more different quantities to find the relationship between them (for example, simultaneous measurements of the increment in the length of a sample depending on changes in its temperature and determination of the linear expansion coefficient using the formula k= l/(l Dt)).
Joint measurements are practically no different from indirect ones.
3.1.3. By connection with the object into contact and non-contact , at which the sensitive element of the device is brought into contact or not brought into contact with the measurement object.
3.1.4. According to accuracy conditions into equal and unequal.
Equal precision measurements – a series of measurements of any quantity made by measuring instruments of equal accuracy under the same conditions.
Unequal measurements– a series of measurements of any quantity, performed by measuring instruments of different accuracy and under different conditions. For example, the mass fraction of water in dried fish was determined by two methods: drying at a temperature of 130 O C and on the HF device at a temperature of 150 O C, the permissible error in the first case is +1%, in the second – +0.5%.
3.1.5 By the number of measurements in a series of measurements single and multiple.
Single measurement– measurements performed once (measurement of a specific time using a clock).
Multiple measurement– a measurement of a physical quantity of the same size, the result of which is obtained from several successive measurements, i.e. consisting of a series of single measurements. Typically, multiple measurements are those that are made more than three times. The arithmetic mean of individual measurements is usually taken as the result of multiple measurements.
3.1.6. According to metrological purpose for technical, metrological;
Technical Dimension– a measurement performed using a working measuring instrument for the purpose of monitoring and managing scientific experiments, monitoring product parameters, etc. (measuring temperature in a smoking oven, determining the mass fraction of fat in fish).
Metrological measurement– a measurement made using a standard and standard measuring instruments for the purpose of introducing a new unit of physical quantity or transferring its size to working measuring instruments.
3.1.7 By expressing the measurement result into absolute and relative;
Absolute measurement– a measurement based on direct measurements of one or more basic quantities and on the use of physical constants. For example, the measurement of gravity is based on the measurement of the basic quantity - mass (m) and the use of the physical constant g: F = mg.
Relative dimension– a measurement made with the aim of obtaining the ratio of a quantity to a quantity of the same name, which plays the role of a unit, or measuring a quantity in relation to a quantity of the same name, taken as the initial one. For example, measuring relative air humidity.
3.1.8. Based on existing sets of measured values on electrical ( current, voltage, power) , mechanical ( mass, number of products, effort); , thermal power(temperature, pressure); , physical(density, viscosity, turbidity); chemical(composition, chemical properties, concentration) , radio engineering etc.
Analysis of the state of measurements in the food industry made it possible to establish the qualitative and quantitative composition of the fleet of measuring equipment, which is characterized by the following ratio (%):
– thermal measurements – 50.7;
– mechanical measurements – 30.4;
– electric power – 12.1;
– physical and chemical measurements – 6.2;
– time and frequency measurements – 0.6.
Principles and methods of measurement
Measuring principle– a physical phenomenon or effect underlying measurements. For example, measuring temperature with a liquid thermometer is based on the increase in volume of liquid as temperature increases.
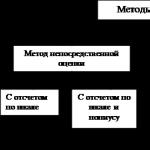
Measurement methodth- a technique or set of techniques for comparing a measured physical quantity with its unit in accordance with the implemented measurement principles.
The classification of measurement methods is presented in Fig. 3.1.

Figure 3.1. Classification of measurement methods
Direct assessment method– a measurement method in which the value of the measured quantity is determined directly from the reading device of a direct-acting measuring device (with reading on a scale or on a vernier scale - an auxiliary scale on which fractions of division of the main scale are counted). For example, counting by a clock or a ruler.
Comparison method with measure– a measurement method in which the measured value is compared with the value reproduced by the measure.
Measure– SI designed to reproduce PV of a given size
The comparison method happens zero, differential, substitution.
Null method– a type of differential method in which the resulting effect of the influence of quantities on a comparison device is brought to zero (cup scales). In this case, the value of the measured quantity is equal to the value that the measure reproduces.
At differential method the measured value x is compared directly or indirectly with the value x and a reproducible measure. The value of x is judged by the difference Δx = x – x m measured by the device in the simultaneously measured values x and xm and by the known value xm reproduced by the measure. Then
x = x m + Δx
Substitution method- a method in which the desired quantity is replaced by a measure with a known value.
Depending on the contact with the measured value, methods are divided into contact and non-contact , at which the sensitive element of the device is brought into contact or not brought into contact with the measurement object. An example of a contact measurement is measuring the temperature of a product with a thermometer, and a non-contact measurement is measuring the temperature in a blast furnace with a pyrometer.
Depending on the principle underlying the measurement, methods are divided into physical, chemical, physicochemical, microbiological, biological .
Physical method– the method is based on recording an analytical signal that records a certain property as a result of a physical process.
Using the physical method, the physical properties of hydrobionts (mass, length, color) and many technological process control parameters (temperature, pressure, time, etc.) are determined. When conducting research, various measuring instruments are used. This method is the most objective and progressive.
Advantages – speed of determination, accuracy of results
Disadvantages - the inability to determine many indicators, mainly analytical
Chemical method– based on recording an analytical signal arising as a result of a chemical reaction, used to assess the composition and properties of a product. For example: titrometry (determination of salinity, gravimetry - determination of sulfate content in table salt).
Advantages: most accurate and objective.
Disadvantages: duration of analysis, requires preparation of reagents, large amount of glassware.
Physico-chemical method– is based on recording a signal that arises as a result of a chemical reaction, but which is also recorded in the form of a measurement of some physical property. Is currently the most progressive. Physico-chemical methods are divided into:
ABOUT optical methods– the connection between the optical properties of the system and its composition is used.
- calorimetric If - based on measuring the absorption of electromagnetic energy in a narrow range of light wavelengths (determining the amount of phenols, vitamin content, etc.).
- refractometric – based on measuring the refractive index of a solution (determination of dry matter content in a tomato).
- potentialometric– is based on determining the equilibrium potential (measuring EMF) and finding the relationship between its value and the potential-determining component of the solution (Determination of pH of a solution)
- polarographic– based on determining the dependence of the current on the increase in voltage on the electrode of a cell immersed in a solution (determination of heavy metals)
- conductometric– based on determining the electrical conductivity of electrolyte solutions (determination of heavy metals, concentration of surface salt in solution).
- combined methods-based on the separation of complex mixtures into individual components and their quantitative determination, there are: chromatographic (thin-layer - determination of fatty acid composition; gas-liquid - determination of amino acid composition, pesticides, adsorption, ion exchange).
Lecture outline:
1 Classification of measurements
2 Physical quantities. Classification of physical quantities
3 Basic measurement equation. Measurement conversion
4 Postulates of measurement theory
5 Testing and control, measurement limits
Classification of measuring instruments can be carried out according to the following criteria.
1. Accuracy characteristics measurements are divided into equal and unequal.
Equal-precision measurements a physical quantity is a series of measurements of a certain quantity made using measuring instruments (MI) with the same accuracy under identical initial conditions.
Unequally accurate measurements physical quantity on is a series of measurements of a certain quantity made using measuring instruments with different accuracy and (or) under different initial conditions.
2. By number of measurements measurements are divided into single and multiple.
Single measurement is a measurement of one quantity made once. In practice, single measurements have a large error; therefore, to reduce the error, it is recommended to perform measurements of this type at least three times, and take their arithmetic average as the result.
Multiple measurements is a measurement of one or more quantities performed four or more times. A multiple measurement is a series of single measurements. The minimum number of measurements at which a measurement can be considered multiple is four. The result of multiple measurements is the arithmetic average of the results of all measurements taken. With repeated measurements, the error is reduced.
3. By type of change in value measurements are divided into static and dynamic.
Static measurements- These are measurements of a constant, unchanging physical quantity. An example of such a time-constant physical quantity is the length of a land plot.
Dynamic measurements- These are measurements of a changing, non-constant physical quantity.
4. By purpose measurements are divided into technical and metrological.
Technical measurements- these are measurements performed by technical measuring instruments.
Metrological measurements are measurements made using standards.
5. By presentation method measurement results are divided into absolute and relative.
Absolute measurements- these are measurements that are performed through direct, direct measurement of a fundamental quantity and (or) the application of a physical constant.
Relative measurements are measurements in which the ratio of homogeneous quantities is calculated, with the numerator being the quantity being compared, and the denominator being the basis of comparison (unit). The measurement result will depend on what value is taken as the basis of comparison.
6. By methods receiving measurement results are divided into direct, indirect, cumulative and joint.
Direct measurements- these are measurements performed using measures, i.e. the measured quantity is compared directly with its measure. An example of direct measurements is the measurement of an angle (a measure - a protractor).
Indirect measurements- These are measurements in which the value of the measurand is calculated using values obtained through direct measurements and some known relationship between these values \u200b\u200band the measurand.
Aggregate Measurements- these are measurements, the result of which is the solution of some system of equations, which With left from equations obtained as a result of measuring possible combinations of measured quantities.
Joint measurements- these are measurements during which at least two inhomogeneous physical quantities are measured With the purpose of establishing the existing dependence between them.
All objects of the surrounding world are characterized by their properties. A property is a philosophical category that expresses such an aspect of an object (phenomenon, process) that determines its difference or commonality with other objects (phenomena, processes) and is revealed in its relations to them. Property – quality category. To quantitatively describe various properties of processes and physical bodies, the concept of quantity is introduced. Magnitude is a property of something that can be distinguished from other properties and assessed in one way or another, including quantitatively. A quantity does not exist on its own; it exists only insofar as there is an object with properties expressed by a given quantity. Ideal quantities mainly relate to mathematics, and are a generalization (model) of specific real concepts. They are calculated in one way or another.
Many properties, in addition to the equivalence relation, also manifest themselves in relation to the presence of a quantitative ordinate of the property - intensity. When an object is divided, such properties usually do not change and are called intensive quantities. By comparing intensive values, one can determine their ratio and order them according to the intensity of a given property. When comparing intensive quantities, an order relation (greater, less or equal) is revealed, i.e. the relationship between the quantities is determined. Examples of intensive quantities are the hardness of a material, odor, etc. Intensive quantities can be detected, classified by intensity, subjected to control, and quantified by monotonically increasing or decreasing numbers. Based on the concept of “intensive quantity,” the concepts of physical quantity and its size are introduced. The size of a physical quantity is the quantitative content in a given object of a property corresponding to the concept of a physical quantity.
Intensive quantities are displayed by quantitative, mainly expert, evaluation, in which properties with a larger size are displayed in higher numbers than properties with a smaller size. Intensive quantities are assessed using order and interval scales, discussed below.
Objects characterized by intense quantities can be subject to control. Control is a procedure for establishing correspondence between the state of an object and the norm. To implement the procedure for the simplest single-parameter control of property X, model objects are needed that characterize parameters equal to the lower X n and upper X, respectively, within the normal limits, and a comparison device. The control result Q is determined by the following equation: below normal (X<Х н); норма (X>X n and X<Х в); выше нормы (X>X c).
If a physical quantity manifests itself in the relations of equivalence, order and additivity, then it can be: detected, classified, controlled and measured. These quantities, called extensive, usually characterize the physical material or energetic properties of an object, for example, body mass, electrical resistance of a conductor, etc. When measuring an extensive quantity, an uncountable set of its dimensions is mapped onto a countable subset in the form of a set of numbers Q, which must also satisfy the equivalence relations, order and additivity. Q numbers are measurement results and can be used for any mathematical operation. The set of such numbers Q must have the following properties:
In order to manifest itself in relation to equivalence, a set of numbers Q representing homogeneous quantities of different sizes must be a set of identically named numbers. This name is a unit of physical quantity or its fraction. A unit of physical quantity [Q] is a physical quantity of a fixed size, which is conventionally assigned a numerical value equal to one. It is used for the quantitative expression of homogeneous physical quantities.
To manifest itself in the relations of equivalence and order, the number q 1, reflecting the larger value Q 1 >Q 2, is chosen larger than the number q 2, representing the smaller value Q 2. In both cases, one unit of physical quantity is used. To satisfy this condition, an ordered set of real numbers with a natural order relation is chosen as the desired set q 1 ,…, q n.
To manifest itself in the relations of equivalence, order and additivity, an abstract number equal to the estimate of the total measurable quantity Q resulting from the addition of the components of homogeneous quantities Q i must be equal to the sum of the numerical estimates qi of these components. The sum of the named numbers Q i reflecting the components must be equal to the named number Q reflecting the total value:
If the condition [Q] = is implemented, i.e. there is equality in the size of the units of all named numbers reflecting the total value Q and its components Q i , then in this case the following concepts are introduced:
The value of a physical quantity Q is an estimate of its size in the form of a certain number of units accepted for it;
The numerical value of a physical quantity, q is an abstract number expressing the ratio of the value of a quantity to the corresponding unit of a given physical quantity.
The equation Q = q[Q] is called the basic measurement equation. The essence of the simplest measurement is to compare the size of the physical quantity Q with the size of the output quantity of the adjustable multivalued measure q[Q]. As a result of comparison, it is established that q[Q] The condition for implementing the elementary direct measurement procedure is to perform the following operations: Reproduction of a physical quantity of a given size q[Q]; Comparison of the measured physical quantity Q with the reproducible measure quantity q[Q]. Thus, based on the use of general postulates of equivalence, order and additivity, the concept of direct measurement has been obtained, which can be formulated as follows: measurement is a cognitive process consisting of comparison through a physical experiment of a given physical quantity with a known physical quantity taken as a unit of measurement. Like any other science, measurement theory is built on the basis of a number of fundamental postulates that describe its initial axioms. A large number of scientific studies are devoted to the construction and study of these axioms-postulates. It should be noted that any attempt to formulate the initial provisions (postulates) of the measurement theory encounters fundamental difficulties. This is due to the fact that, on the one hand, the postulates must represent objective statements, and on the other, the subject of metrology is measurements, i.e. the type of activity people undertake to achieve subjective goals. Consequently, it is necessary to formulate objective statements that would serve as the foundation of a scientific discipline that has a significant subjective element. The first postulate of metrology is postulate a: within the framework of the accepted model of the object of study, there is a certain measurable physical quantity and its true value. If, for example, we assume that the part is a cylinder (the model is a cylinder), then it has a diameter that can be measured. If the part cannot be considered cylindrical, for example its cross-section is an ellipse, then measuring its diameter is pointless, since the measured value does not carry useful information about the part. And, therefore, within the framework of the new model, diameter does not exist. The measured quantity exists only within the framework of the accepted model, i.e. makes sense only as long as the model is considered adequate to the object. Since, for different purposes of research, different models can be compared to a given object, then from the postulate a follows corollary a 1: for a given physical quantity of the object being measured, there are many measured quantities and, accordingly, their true values. So, from the first postulate of metrology it follows that the measured property of a measurement object must correspond to some parameter of its model. This model must allow this parameter to be considered unchanged during the time required for measurement. Otherwise, measurements cannot be taken. This fact is described by postulate b: the true value of the measured quantity is constant. Having identified a constant parameter of the model, you can proceed to measuring the corresponding value. For a variable physical quantity, it is necessary to isolate or select some constant parameter and measure it. In the general case, such a constant parameter is introduced using some functional. An example of such constant parameters of time-varying signals introduced through functionals are rectified average or root mean square values. This aspect is reflected in corollary b1: to measure a variable physical quantity, it is necessary to determine its constant parameter - the measured quantity. Measurements based on the use of human senses (touch, smell, vision, hearing and taste) are called organoleptic. The measurement of time, for example, or gravity (by astronauts) are based on sensations. Even less perfect measurements on the order scale are based on impressions. Measurements based on intuition are called heuristics. Measurements performed using special technical means are called instrumental. These may include automated and automated ones. In automated measurements, the role of a person is not completely excluded (receiving data from the reporting device of a measuring device or digital display). Automatic measurements are performed without human intervention. Their result is presented in the form of a document and is completely objective. Indicators are technical devices designed to detect physical properties. Measuring instruments are all technical means used in measurements and having standardized metrological characteristics. Real measures are designed to reproduce a physical quantity of a given size, which is characterized by the so-called nominal size. Measuring transducers are measuring instruments that produce signals of measuring information in a form convenient for further conversion, transmission, storage, processing, but, as a rule, inaccessible to direct perception by an observer. The unity of measurements is understood as a state in which the results are expressed in legal units, and the accuracy of measurements is documented. Metrological characteristics of measuring instruments are those technical characteristics that affect the results and accuracy of measurements. The measurement scale for a quantitative property is a scale for a physical quantity. A physical quantity scale is an ordered sequence of values of a physical quantity, adopted by agreement based on the results of precise measurements. In accordance with the logical structure of the manifestation of properties, five main types of measurement scales are distinguished. Naming scale (classification scale). Such scales are used to classify empirical objects whose properties appear only in relation to equivalence. These properties cannot be considered physical quantities, therefore scales of this type are not scales of physical quantities. This is the simplest type of scale, based on assigning numbers to the qualitative properties of objects, playing the role of names. An example of naming scales are widely used color atlases designed for color identification. Order scale (rank scale). If the property of a given empirical object manifests itself in relation to equivalence and order in increasing or decreasing quantitative manifestation of the property, then an order scale can be constructed for it. It is monotonically increasing or decreasing and allows you to establish a greater/lesser ratio between quantities characterizing the specified property. In order scales, zero exists or does not exist, but in principle it is impossible to introduce units of measurement, since a proportionality relation has not been established for them and, accordingly, there is no way to judge how many times more or less specific manifestations of a property are. Order scales with reference points marked on them have become widespread. Such scales, for example, include the Mohs scale for determining the hardness of minerals, which contains 10 reference (reference) minerals with different hardness numbers: talc - 1; gypsum - 2; calcium - 3; fluorite - 4; apatite - 5; orthoclase - 6; quartz - 7; topaz - 8; corundum - 9; diamond - 10. The assignment of a mineral to a particular gradation of hardness is carried out on the basis of an experiment, which consists of scratching the test material with a supporting one. If after scratching the test mineral with quartz (7) a trace remains on it, and after orthoclase (6) there is no trace, then the hardness of the test material is more than 6, but less than 7. Evaluation on order scales is ambiguous and very conditional, as evidenced by the considered example. Interval scale (difference scale). These scales are a further development of order scales and are used for objects whose properties satisfy the relations of equivalence, order and additivity. The interval scale consists of identical intervals, has a unit of measurement and an arbitrarily chosen beginning - the zero point. Such scales include chronology according to various calendars, in which either the creation of the world, or the Nativity of Christ, etc. is taken as the starting point. The Celsius, Fahrenheit and Reaumur temperature scales are also interval scales. There are practically two ways to set the scale. In the first of them, two values Q 0 and Q 1 are selected, which are relatively simply implemented physically. These values are called reference points, or main reference points, and the interval is called the main interval (Q 1 -Q 0). Relationship scale. These scales describe the properties of empirical objects that satisfy the relations of equivalence, order and additivity (scales of the second kind are additive), and in some cases proportionality (scales of the first kind are proportional). Their examples are the scale of mass (second kind), thermodynamic temperature (first kind). In ratio scales, there is an unambiguous natural criterion for the zero quantitative manifestation of a property and a unit of measurement established by agreement. From a formal point of view, the ratio scale is an interval scale with a natural origin. All arithmetic operations are applicable to the values obtained on this scale, which is important when measuring a physical quantity. Relationship scales are the most advanced. Absolute scales. Some authors use the concept of absolute scales, by which they mean scales that have all the characteristics of ratio scales, but additionally have a natural, unambiguous definition of the unit of measurement and do not depend on the adopted system of units of measurement. Such scales correspond to relative values: gain, attenuation, etc. To form many derived units in the SI system, dimensionless and counting units of absolute scales are used. Note that the scales of names and order are called non-metric (coceptual), and the scales of intervals and ratios are called metric (material). Absolute and metric scales belong to the category of linear. The practical implementation of measurement scales is carried out by standardizing both the scales and measurement units themselves, and, if necessary, the methods and conditions for their unambiguous reproduction. Control questions: 1 Define a physical quantity. Give examples of quantities belonging to different groups of physical processes. 2 What are extensive and intensive physical quantities? What are their similarities and differences? Give examples of physical quantities of each type. 3 What is a physical quantity scale? Give examples of different scales of physical quantities. 4 Name the main operations of the measurement procedure. Tell us how they are implemented when measuring the size of a part with a caliper. 5 Give examples of measuring transducers, multivalued measures and comparison devices used in measuring instruments known to you. 6 What is a measuring instrument? Give examples of measuring instruments for various physical quantities.